Method description
Depending on the nature of the environment, the heat capacity can be measured via (semi-)adiabatic method or modulation calorimetry. The former one is usually used when the sample is measured as-is, whereas the latter one is typically used when the sample is placed in a pressure medium.
The adiabatic method can be used in two complementary scenarios:
- heat pulse calorimetry - sample is heated and relaxation to bath temperature is observed, the temperature relaxation time constant is given by the ratio between thermal conductance and total heat capacity, accessible time constants are limited by the readout electronics (units of seconds) and stability of bath temperature (hundreds of seconds), a point-by-point method for suited for detailed investigations in larger temperature intervals.
- dual-slope method - similar to the one above, in our case using significantly larger heat pulses to pro larger temperature intervals for quick surveys in unknown systems, self-correcting for parasitic leaks. An excellent sample equilibrium within the system (sample-sample holder-sample thermometer) is needed. Due to larger pulses is it suitable for investigation of the first-order phase transition.
On the other hand, the AC calorimetry does rely on the heat link and can be used when the sample is emerged in medium (e.g. pressure cell). A thermocouple is placed on the sample and the sample’s response to the oscillatory heating is observed. In a certain frequency window, the heat capacity is inversely proportional to the amplitude of temperature oscillations.
Where to use it?
Heat capacity method is available in the following cryostats:
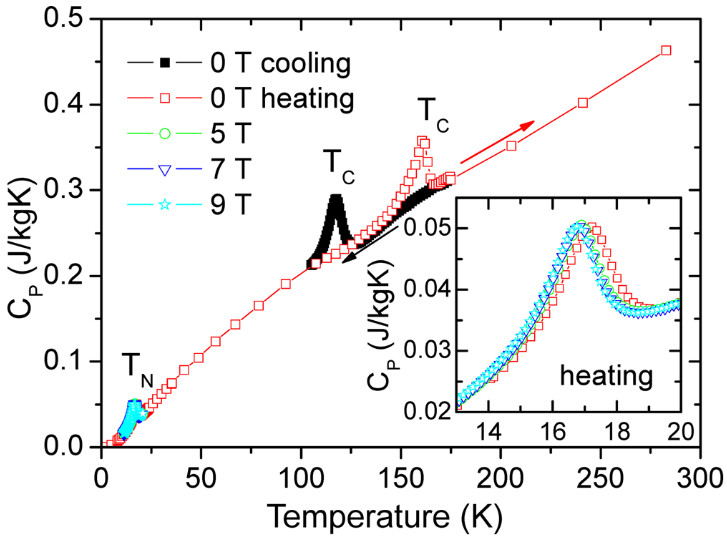
Fig. 1: Temperature and magnetic field dependence of heat capacity of Pb2MnTeO6. Large thermal hysteresis of TC is seen.TN exhibits 0.4 K shift down with an applied magnetic field. Inorg. Chem. 2016, 55, 9, 4320–4329
Fig. 2: LEFT: Temperature dependence of the heat capacity of the VI3 single crystal at fields 0 to 18.3T. RIGHT: Resulting magnetic phase diagram of given transition.